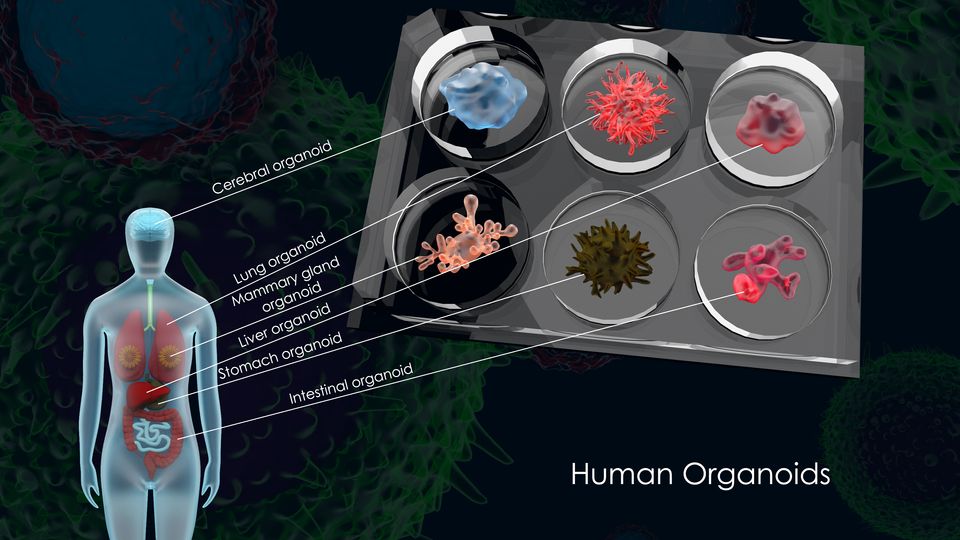
Human 3D organoids are in vitro 3D cultures that mimic the structure and function of human organs. They are derived from stem cells, which can self-renew and differentiate into various cell types. [1] These organoids offer a powerful platform for disease modelling, drug development, personalised treatment, and regenerative medicine.
As Xu et al said in 2018 [2], organoid technology bridges the gap between conventional two-dimensional cell line culture and in vivo models. The near-physiological technology can virtually recapitulate organ development and human diseases, such as infectious diseases, genetic abnormalities and even cancers. In addition, organoids can more accurately predict drug responses and serve as an excellent platform for drug development, including efficacy evaluation, toxicity testing and pharmacokinetics analysis.
Ark Invest interviewed Professor Thomas Hartung, a leading expert in biomedical innovation at Johns Hopkins Bloomberg School of Public Health. [3] They explored how human organoids—3D organ proxies—are revolutionising drug discovery and toxicology testing by reducing reliance on animal models. They show advancements in stem cell science, microfluidics, and artificial intelligence are converging to accelerate clinical trials, increase safety, and lower costs. The conversation also touches on the ethical considerations of biological computing, where organoids may one day aid in cognition and pattern recognition tasks.
Ethics Considerations
Hartung describes how the original stem cell research changed when they moved from stem cells derived from human eggs to skin cells in 2006. It was a breakthrough, and the current Human Atlas project will enable over 2000 cell types to be created. John Hopkins already generates 10,000 human brain cells a week for experimentation, saving hundreds of animal lives. Cancer and drug studies are now done in vitro and bypass the requirement for animal testing. Why test on animals, rats or monkeys when you can test on human organoids and cut out the intermediary step from animals to humans? Organoids derived from a human with cancer, for example, have the exact genetic makeup you want to screen or test the new compound on.
Creation Methodologies
Generating organoids involves mimicking the organ development process from pluripotent stem cells in vitro [4]. While it’s challenging to replicate all the biochemical cues of embryonic development precisely, cells in vitro tend to follow a semi-autonomous differentiation trajectory. A common approach involves embedding stem cells in a 3D medium, such as Matrigel or Cultrex BME, a laminin-rich extracellular matrix [5] (Wiki – Organoid) . When using PSCs, the cells often form embryoid bodies before differentiating into organ-specific structures. Advanced techniques like 3D bioprinting are also being explored to create complex organ precursors with vascular systems [6], even incorporating human cardiomyocytes derived from induced PSCs to construct endothelialized myocardium. ([7]
Applications
When we started biology, we used animals for clinical and research purposes. Over three times more animals were used back in the 1970s for testing. Originally stem cells were used but they looked like eggs in a pan. Now, with new techniques, they look much like human tissue, with the advent of 3D printing and understanding cell types. Alternative tools using human organoids now outperform animal studies. Animals do not always behave in the same way.
For example lung tissue may have 40 different types of cells. When you test, you really want all cell types there to be more representative of the lung.
Furthermore, cell cultures started with a nutrient solution supply of O2 etc. It is not a homeostatic solution. Within 2 days, that nutrient solution will have to be changed completely. It is counter productive to the research. We also have been using cancer cell types to keep the cell lines going which means the results can be less representative.
About ½ of new drugs are human proteins, which lends to the new techniques.
Clinical Utilities
Organoids hold immense potential for clinical applications. Patient-derived organoids, particularly from cancer tissues, enable personalised medicine by allowing drug screening and testing of treatment strategies tailored to individual patients. [2] [1] They can also be used to study disease processes, such as endometrial cancer [8] and other pathologies like endometriosis and infertility.
Human 3D Oganoids Accelerate Drug Development
The first human organoid developed for pharmaceutical use in 2019 is already in phase 2 Clinical trials. Typically that process takes up to 12 years.
Science is accelerating. Conventionally, over 10,000 compounds are tested to get a product to take forward. Only 10 of the 10,000 go into clinical trials, and the cost of the final product is over $2 billion. Now, with AI and omics might use cancer cells that have that receptor. Development needs a more complex approach. But the payback is that one day earlier is worth about $1 million revenue based on 8 years of patent protection following the 14 years of development.
During the pandemic we skipped animal testing. Yet within 2 years, we had 9 successful vaccines and 2 antivirals on the market. The secret of the virus was the genetic understanding of the virus and testing on organoids.
The FDA changed its rules in April 2024 with the new methodology as it is more efficient and avoids the whole animal testing regime.
In pharmaceutical research, organoids provide a valuable tool for drug discovery and development. They offer a more accurate representation of human tissues compared to traditional cell lines, allowing for more reliable drug screening and toxicity testing. [8] Organoids can also be used to study drug metabolism and efficacy, leading to the development of more effective and targeted therapies. For instance, liver organoids can be used for in vitro drug screening and liver disease studies [6]
Roche, Astra Zeneca and Sanofi are using the techniques. Some have campuses with over 400 staff working. Had 8 cases already that have skipped to registration, without any animal data
AI Disruptive Technology.
Drug development has been turned on its head as discussed on this article. Most drug companies were early users of AI since 2019. In the five years since 2019, 18 drugs are under development. One is already in in phase 2A in 2024 and now in phase 2B. That is unheard of. See the blog post on impacts of AI in drug discovery and clinical trials.
Human Computers
Our human brain of 1.4kg has a computing capacity of 1 exaflop. It is a supercomputer and only matched by a supercomputer in Kentucky in June 2022 for 68m2 $60 million and 2.5 petabytes of memory. We have all that on our shoulders. Our brains learn on the fly, whereas machines need updating.
Growing brain cell organoids opens up the opportunity to build thinking machines. It arose from autism research. Generating brain organoid costs less than cent each, versus animals at $40. Now brain organoids are in parallel with animal studies. While it is a way off, and techniques have not been developed yet, but when one considers a human brain that is 10mm in size has some thinking processes.
References
[1] A. Semertzidou, J. J. Brosens, I. McNeish, and M. Kyrgiou, ‘Organoid models in gynaecological oncology research’, Cancer Treat. Rev., vol. 90, Nov. 2020, doi: 10.1016/j.ctrv.2020.102103.
[2] H. Xu, Y. Jiao, S. Qin, W. Zhao, Q. Chu, and K. Wu, ‘Organoid technology in disease modelling, drug development, personalized treatment and regeneration medicine’, Exp. Hematol. Oncol., vol. 7, no. 1, p. 30, Dec. 2018, doi: 10.1186/s40164-018-0122-9.
[3] ‘Replacing Animal Testing: Organoids And AI With Thomas Hartung’. Accessed: Feb. 04, 2025. [Online]. Available: https://www.ark-invest.com/podcast/replacing-animal-testing-organoids-and-ai-with-thomas-hartung
[4] J. Kim, B.-K. Koo, and J. A. Knoblich, ‘Human organoids: model systems for human biology and medicine’, Nat. Rev. Mol. Cell Biol., vol. 21, no. 10, pp. 571–584, Oct. 2020, doi: 10.1038/s41580-020-0259-3.
[5] ‘Organoid’, Wikipedia. Dec. 07, 2024. Accessed: Feb. 04, 2025. [Online]. Available: https://en.wikipedia.org/w/index.php?title=Organoid&oldid=1261763475
[6] C. S. Ong et al., ‘3D bioprinting using stem cells’, Pediatr. Res., vol. 83, no. 1, pp. 223–231, Jan. 2018, doi: 10.1038/pr.2017.252.
[7] Y. S. Zhang et al., ‘Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip’, Biomaterials, vol. 110, pp. 45–59, Dec. 2016, doi: 10.1016/j.biomaterials.2016.09.003.
[8] A. Katcher et al., ‘Establishing patient-derived organoids from human endometrial cancer and normal endometrium’, Front. Endocrinol., vol. 14, Apr. 2023, doi: 10.3389/fendo.2023.1059228.